FD&C Act SEC. 587 Definitions
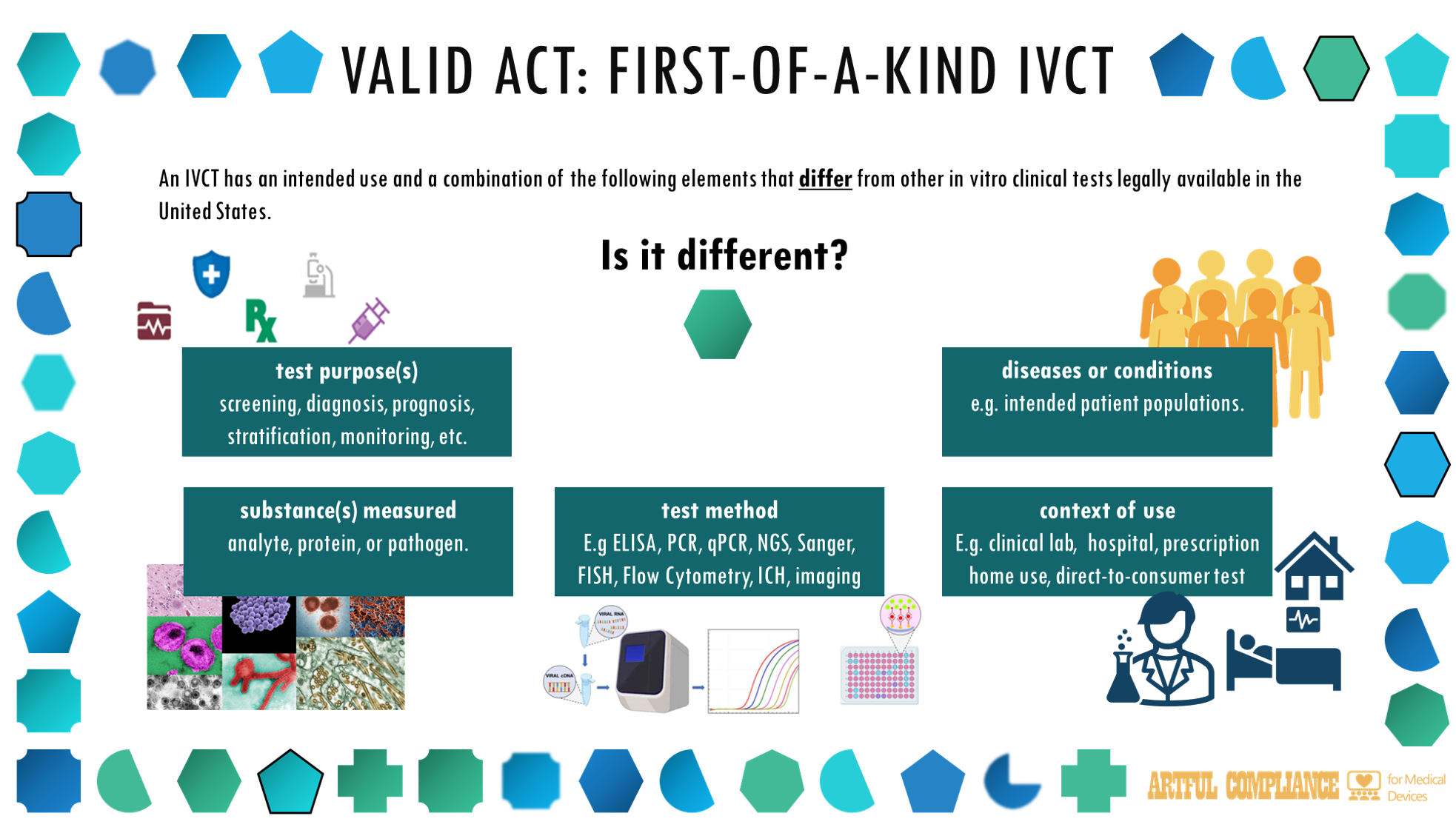
“(8) FIRST-OF-A-KIND.—The term ‘first-of-a-kind’ means, with respect to an in vitro clinical test, a test that has an intended use and a combination of the elements specified in paragraph (10) that differ from the intended use and such elements of other in vitro clinical tests that already are legally available in the United States.
“(10) INDICATIONS FOR USE.—The term ‘indications for use’ means one or more in vitro clinical tests that have all of the following notification elements in common:
“(A) Substance or substances measured by the in vitro clinical test, such as an analyte, protein, or pathogen.
“(B) Test method.
“(C) Test purpose or purposes, as described in section 201(ss)(1)(A).
“(D) Diseases or conditions for which the in vitro clinical test is intended for use, including intended patient populations.
“(E) Context of use, such as in a clinical laboratory, in a health care facility, prescription home use, over-the-counter use, or direct-to-consumer testing.
Recent Comments